Lupine Publishers| Potentiation of Activity of Benfotiamine Co Administered with Thyroxine in Diabetes Induced Peripheral Neuropathy in Rats
Abstract
Diabetic peripheral neuropathy (DPN) is a multi-etiological microvascular complication. Prolong hyperglycemia leads to formation of advance glycation end product (AGE) and oxidative stress which are contributors of nerve dysfunction. DPN manifests as pain, slowing of nerve conduction velocity (NCV), sensory loss etc. The aim of the present study is to evaluate the individual and combined protective effect of benfotiamine (BT) and thyroxine (T4) against Streptozotocin (STZ) induced DPN in rats. After 48 hours of a single injection of STZ (60 mg/kg bw i.p) diabetic rats were administered BT (100 mg/kg p.o.), T4 (1mg/kg.s.c,) and their combination. Diabetic rats at 5th week, exhibited significant decrease in body weight, hyperalgesia, decreased muscle coordination, grip strength and NCV. Antioxidant activity of reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD) was also found to be significantly decreased. Significant higher levels of glycosylated hemoglobin (GHb) and Malondialdehyde (MDA) were also observed in diabetic rats. Treatment with BT, T4 and their combination attenuated the decrease in level of nociceptive threshold, muscle coordination, grip strength.
NCV and antioxidant activity. Significant decrease in the elevated levels of GHb and MDA was also observed. A histopathological study of sciatic nerve also confirmed the improvement in cell architecture as compared to diabetic rats and has strengthened the neuroprotective effect of BT and T4 combination group. An improved In Vitro AGE inhibitory activity of BT, T4 and their combination was observed. These finding suggested that BT, T4 and their combination exerts a protective effect in progression of diabetic neuropathy by decreasing GHb, AGE formation and oxidative stress.
Keywords:Micro vascular; NCV; Antioxidant; AGE; Thyroxine; Benfotiamine
Abbreviations:DM: Diabetes mellitus; DPN: Diabetic Peripheral Neuropathy; MAPK: Mitogen Activated Protein Kinase; NCV: Nerve Conduction Velocity; TH: Thyroid Hormones; BT: Benfotiamine; AGE: Advance Glycation End Product; LPO: Lipid Peroxidation; SOD: Superoxide Dismutase; CAT: Catalase; GSH: Glutathione; TBARS: Thiobarbituric Acid Reactive Substances; BSA: Bovine Serum Albumin; PKC: Protein Kinase C; ROS: Reactive Oxygen Species
Introduction
Diabetes mellitus (DM) is a worldwide major health problem and it is a chronic metabolic disorder characterized by hyperglycemia resulting from inadequate secretion or impaired action of endogenous insulin. The prevalence of diabetes is increasing worldwide and is believed to increase to 300 million by the year 2030 [1]. Uncontrolled or persistent hyperglycemia in DM leading to several microvascular and microvascular complications. Diabetic peripheral neuropathy (DPN) is a common microvascular complication of DM affecting more than 50% of the diabetic patients [2]. The pathogenesis of DPN is considered to be complex and multifactorial resulting from contributions of various pathways including metabolic and vascular factors, which consists of activation of polyol pathway, advanced glycation end products pathway, hexosamine pathway, increased activity of mitogen-activated protein kinase (MAPK), protein kinase C, poly (ADP-ribose) polymerase, oxidative stress, apoptosis, impaired neurotrophic support, autoimmunity, inflammation, up regulation of endothelin [3]. The impairment of nerve function is a well-established early manifestation of diabetes both clinically and in experimental animal models. DPN causes dysfunction of small and large nerve fibers and negatively impacts quality of life in diabetic patients. Small-fiber peripheral neuropathy is characterized by behavioral abnormalities (cold, thermal hyperalgesia, loss of grip strength and motor incoordination and burning or lancinating pain, and predisposition to foot ulceration). Large-fiber dysfunctions include loss of position and vibration sensation, nerve-conduction abnormalities, and distal muscle weakness. Early disorders of nerve function include slowing in nerve conduction velocity (NCV), followed by axonal degeneration, axoglial disjunction, paranodal and loss of fibre density. Microangiopathy [4]. A number of different agents from diverse chemical classes have entered clinical trials for the treatment of metabolic abnormalities in DPN, but only few approved for clinical use while other drugs either ineffective or withdrawn [2]. Current treatment options for symptomatic treatment of DPN include antidepressants, anticonvulsants. These agents are modestly effective for symptomatic relief, but they neither affect the underlying pathology nor do they slow progression of the disease [5]. Hence a novel approach to bridge the gap in selecting the compound in treatment of DPN was used .The discovery of use of a drug for a new indication is an arbitrary process, as shown by many past examples like the use of zinc acetate for the treatment of Wilson’s disease [6], arsenic for acute promyelocytic leukemia [7], amphotericin B for leishmaniasis [8], and thalidomide for multiple myeloma [9]. The discovery of these “alternative” uses for drugs different from originally intended drug development process is referred to as drug repurposing or repositioning. Repositioning of drug efforts has many advantages, because the pharmacokinetics and pharmacodynamics of the drug are known, repositioning discoveries are less costly and quicker than traditional discovery efforts [10], which usually take 10-15 years [11], and cost upward of $1 billion [12], Although physicians and pharmaceutical/ biotechnology companies have manual methods and prior knowledge that enable them to carry out drug repositioning clinical trials, such successful repositioning of drugs is often serendipitous and rare. In this study we have selected thyroxine to explore for its activity in DPN. Thyroid hormones (TH) [T4 (tetraiodothyronine) and T3 (triiodothyronine)], the only iodine-containing compounds with biological activity [13]. TH stimulate synthesis of Na+/K+ ATPase and also regulates metabolism by stimulating protein synthesis and increase the use of glucose and fatty acids for ATP production. They also increase lipolysis and enhance cholesterol excretion [14]. The cardiac side effect of D isomer of thyroxine resulted discontinuation of the clinical uses of this hormone. Under normal conditions, about 41%of thyroxine is converted to T3, and about 21% is converted to metabolically inactive 3,3,5-triiodothyronine (reverseT3, rT3). T3 is a powerful inducer of pancreatic acinar cell proliferation in rodents [15]. In Vitro studies of human and rodent insulinoma cell lines showed that T3 protected from apoptosis and induces β-cell growth and proliferation [16]. A serendipitous, positive association between serum-FT3 (free tri iodothyronine) and an estimate of insulin production was found in euthyroid, lean, healthy individuals [17].Treatment of Human pancreatic duct cells (hPANC-1)with T3 induced changes in cell morphology, promotes cell differentiation into insulin-producing β-cells, unregulated insulin and glucose transporter protein-2 transcripts, and increases insulin release into the medium. TH receptor has been identified in pancreatic β -cell lines [18,19], T3-enhanced insulin release in isolated rat pancreatic islets exposed to glucose concentrations of 2-8 mmol/l, had no effect at concentrations of 12 mmol/l, and inhibited insulin release at concentrations of 16.6 mmol/l [20]. T3 promoted expression of important proteins involved in both glucose and lipid metabolism that may influence insulin secretion [21]. Benfotiamine (BT), a lipid soluble vitaminB1 with much higher bioavailability than thiamine [5] Benfotiamine was shown to prevent experimental diabetic retinopathy and In Vitro hyperglycemia-induced endothelial dysfunction. The effects of benfotiamine on in vivo endothelial function remained unknown [22]. Therefore, the present study was designed to evaluate whether diabetes induced DPN can be reversed by treatment with thyroxine and BT.
Materials and Methods
Experimental Animals
In-house laboratory bred healthy Wistar rats of weighing 200-250g were included for the study. Animals were housed in polypropylene cages on clean paddy husk bedding. Animals were maintained under controlled temperature at 25°C± 2°C with 12hr light/dark cycle with food and water provided ad labium. Animals which do not comply with above criteria, and which are found to be diseased will be excluded from the study. Before conducting the experiment, ethical clearance was obtained from “Institutional animal ethics committee” Al-Ameen College of Pharmacy”, Bangalore. Approval No: AACP/P-48.
Drugs and Reagents
Thyroxine (gift sample from Apotex Pharmachem India Pvt. Ltd.), benfotiamine (gift sample from Strides Arco Lab Pvt.Ltd.) were used in the present investigation. Streptozotocin (STZ) was purchased from Sigma Aldrich. Commercial diagnostic kit for the estimation of serum glucose was obtained from Span Diagnostics Ltd. Glycosylated hemoglobin kit was obtained from Crest Biosystems Pvt. Ltd. Other chemicals and reagents were of analytical grade and purchased from local suppliers.
Design of the Experiment
Induction of Diabetic Peripheral Neuropathy: After an overnight fast, Wistar rats were administered a single injection of streptozotocin [60 mg/kg of body weight i.p.in 100 mM sodium citrate buffers, pH 4.5] [23]. After 48 hours, animals with fasting blood glucose levels higher than 250mg/dl were selected for the study.
Experimental Procedure
Rats were randomly assigned in six groups (n=6)
Group1: Normal Control
Group2: Diabetic Control
Group3: Diabetic Control+ T4 (1mg/kg.s.c) [24] thrice a week for 5 weeks
Group4: Diabetic Control+ BT (100 mg/kg p.o.) [25] daily for 5 weeks
Group5: Diabetic Control +BT (100 mg/kg p.o.)+T4 (1mg/ kg.s.c,) for 5 weeks
For preventive studies treatment was started from day 2 of STZ administration along with insulin (3IU/kg, s.c) [26]. 5 weeks post treatment various behavioral, biochemical, electrophysiological and histopathological parameters were studied.
Behavioral Studies
Thermal and Cold Hyperalgesia [27,28]: Thermal and cold hyperalgesia were measured using the tail-immersion test in water maintained at high (46ºC) or low (4ºC) temperature. The duration of tail immersion was recorded, and a cut-off time of 15 s was used.
Measurement of Muscle Incoordination Using Rota Rod [28,29]: Rota rod has been used to evaluate motor coordination by testing the ability of rats to remain on a revolving rod. The apparatus has a horizontal rough metal rod of 3 cm diameter attached to a motor with variable speed. This 70 cm long rod was divided into four sections by wooden partitions. The rod was placed at a height of 50 cm to discourage the animals to jump from the rotating rod. The rate of rotation was adjusted in such a manner that it allowed the normal rats to stay on it for 5 min. Each rat was given five trials before the actual reading was taken. The readings were taken at15, 25, rpm after treatment in all groups of rats.
Measurement of grip strength [29,30]: Grip strength meter was used for evaluating grip strength of animals. Before commencement of experiment, the animals were acclimatized by placing on the instrument for some time to train, and then rats were held by the tail above the grid of grip strength meter. The animal was moved until its front legs were grasped the grid and it was brought to an almost horizontal position. The base of the tail was then pulled following the axle of the sensor until it released the grid. The force achieved by the animal was then displayed on the screen and was recorded as new tons or kg units.
Biochemical Studies
Estimation of GHb [30,31]: At the end of study (5 weeks) blood was withdrawn through retroorbital plexus of overnight fasted rat under light ether anesthesia using a glass capillary and collected in EDTA tubes. The glycosylated hemoglobin was determined by using kit. A hemolysed preparation of whole blood is mixed continuously for 5 minutes with a weakly binding cation-exchange resin. The labile fraction is eliminated during the hemolysate preparation and during the binding. During this mixing, HbAo binds to the ion exchange resin leaving GHb free in the supernatant. After the mixing period, a filter separator is used to remove the resin from the supernatant. The percent GHb is determined by measuring absorbance of the GHb fraction & the total hemoglobin (THb) fraction. The ratio of the absorbance of the GHb & the THb fraction of the Control and the Test is used to calculate the percent GHb of the sample using below formula.
Preparation of Nerve Homogenate: A segment of sciatic nerve, approximately 1.5 cm in length, 5mm proximal and 5 mm distal was used for preparing the 10% w/v homogenates for biochemical estimation. Tissue homogenates were prepared in 0.1M phosphate buffer (pH 7.4). The homogenate was centrifuged at 1000 rpm 4ºC for 3 min and the supernatant divided into two portions, one of which was used for measurement of lipid peroxidation (LPO) and the remaining supernatant was again centrifuged at 12,000 rpm at 4ºC for 15 min and used for the measurement of lipid peroxidation (LPO), superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH).
Measurement of Lipid Peroxidation: The extent of lipid peroxidation in terms of thiobarbituric acid reactive substances (TBARS) formation was measured according to the method of Esterbauer and Cheeseman. Tissue extracts were mixed separately with 1ml TCA (20%), 2ml TBA (0.67%) and heated for 1h at 100°C, after cooling, the precipitate was removed by centrifugation. The absorbance of each sample was measured at 535 nm using a blank containing all the reagents except the sample. As 99% TBARS are malondialdehyde (MDA), so TBARS concentrations of the samples were calculated using the extinction coefficient of MDA, which is1.56 x 105 M-1 cm-1 and were expressed as μM of malondialdehyde per mg protein [31-34].
Estimation of Superoxide Dismutase (SOD) and Catalase Activity: Sciatic nerve homogenate was centrifuged at 4°C, 17,500×g for 10min. Supernatant was used for the measurement of SOD activity by pyrogallol autooxidation method [35,32] and catalase activity by H2O2 degradation method, which is a quantitative spectroscopic method developed for following the breakdown of H2O2 at 240nm in unit time. The sample readings were taken by placing 1ml of phosphate buffer and 100 μl of tissue homogenate in the reference cuvette and 1 ml of H2O2 and 100 μl of homogenate in the test cuvette in the spectrophotometer. For each measurement, the reading was taken at 240nm 1min after placing the cuvettes in Shimadzu spectrophotometer [36,33].
Measurement of Reduced Glutathione Activity: Reduced glutathione was assayed by the method of Van Dooran [37,34]. Briefly1.0 ml of sciatic nerve homogenate (10%w/v) was precipitated with 1.0 ml of sulphosalicylic acid (4%). The samples were kept at 4°C for at least 1h and then subjected to centrifugation at 1200g for 15min at 4°C.The assay mixture contained 0.1 ml supernatant,2.7 ml phosphate buffer (0.1M, pH 7.4) and 0.2 ml 5,5, dithiopyrs (2-nitro benzoic acid) (Ellman’s reagent, 0.1 mM, pH 8.0) in a total volume of 3.0 ml. The yellow color developed was read immediately at 412nm and the reduced GSH levels were expressed as μg/mg protein.
Electrophysiological Studies
Isolation of Sciatic Nerve: The rats were sacrificed by administration of overdose of ketamine/xylazine i.p. After anesthesia, rat backs were shaved and NCV was recorded. Briefly incision was made at L4-L6 spinal segments. The sciatic nerves were surgically exposed from sciatic notch to the gastrocnemius tendon and the left and right sciatic nerves were rapidly removed, carefully impregnated on fine filter paper to remove any accompanying blood soaked for 10 minutes in Ringer-Locke buffer to prevent spontaneous firing of the nerve.
Measurement of Nerve Conduction Velocity (NCV): The rats were anesthetized by administration of thiopentone sodium, 30mg/kg, and i.p [1]. After anesthesia, rat backs were shaved and motor NCV was recorded. Briefly incision was made at L4-L6 spinal segments. The sciatic nerves were surgically exposed from sciatic notch to the gastrocnemius tendon and the left and right sciatic nerves were rapidly removed, carefully impregnated on fine filter paper to remove any accompanying blood soaked for 10 minutes in Ringer-Locke buffer to prevent spontaneous firing of the nerve [32].The left sciatic nerves were then placed in a moist nerve chamber (MLT016/B AD Instruments, Australia) to measure NCV. NCV was measured by stimulating proximally at the sciatic notch by stimulating electrode (MLA270 AD Instruments, Australia) with 10 mV at 1Hz to 5 Hz and the action potential was measured using recording electrodes (MLA 285) by placing distally to the sciatic knottins was calculated by distance between stimulating and recording electrodes divided by the latency. Right sciatic nerves were transferred into Glutaraldehyde solution for histopathological studies and rinsed with ice cold saline homogenized in chilled phosphate buffer (pH 7.4) and used to assay lipid peroxidation, reduced glutathione and catalase [33,35].
Histopathological Studies
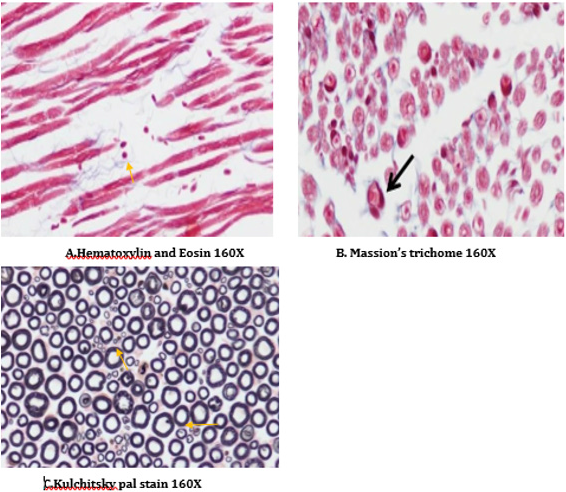
The right sciatic nerves were isolated and transferred in to 0.05mol/L phosphate buffered (30g/L) glutaraldehyde solution for histopathological studies (H&E staining, Kulchitsky Pal staining and Massion’s trichome) [36,37].
To determine In Vitro glycation of protein bovine serum albumin (BSA)-glucose assay was performed based on the procedure of Brownlee et al. BSA (l0mg/mL) was incubated with glucose (500mM) in phosphate buffered-saline (pH 7.4) and extract containing sodium azide (0.02%) at 37ºC with a final concentrations of BSA (2mg/mL), glucose (40mM), sample (0.1 to 0.5mg/mL). Sterilization of reagents and samples were done by filtration through 0.2μm membrane filters. Aminoguanidine was used as an inhibitor positive control and a reactions without any inhibitor were also setup. All the samples and positive control were kept for incubation at 37ºC for 15 days. After 15 days of incubation, fluorescence intensity (excitation wavelength of 370nm and emission wave-length of 440nm) was measured for the test solutions. Percent inhibition was calculated as follows: Inhibition %= Inhibition % = (1− ( As − Ab) ( Ac − Ab) ×100
Where As = fluorescence of the incubated mixture with sample, Ac, Ab = are the fluorescence of the incubated mixture without sample as a positive control and the fluorescence of incubated mixture without sample as a blank control.
Statistical Analysis
Statistical evaluations were done by ANOVA, expressed as mean± S.E.M. followed by Bonferroni comparison test using Graph Pad In Stat (Ver.3.10) and Graph Pad Prism 5 computer programme.
Results
Assessment of General Toxicity
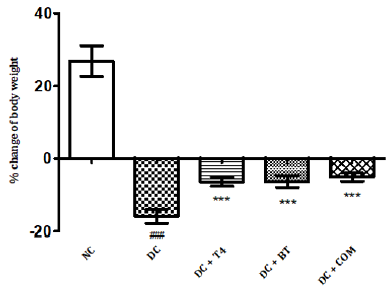
The percentage body weight of normal and diabetic rats treated with T4, BT and combination at 5th week was found to be 26.87±1.74g, -15.90 ± 0.769g,-6.433±0.493g.-6.635 ± 0.661,-5.150± 0.4366.The percentage of change of body weight of diabetic rats significantly less (<P0.001) as compared to normal control, similarly the percentage of change of body weight of diabetic treated rats was significantly less (<P0.001) as compared to diabetic control rats control (Figure 1).
Figure 1: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on %body weight change in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, and *** P<0.001 Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.
Behavioral Studies
Thermal and Cold Hyperalgesia
The tail flick latencies in both hot and cold immersion test of diabetic rats significantly changed at 5th week in diabetic rats as compared to normal rats (P<0.001) . 5 weeks treatment with T4, BT and combination significantly improved P<0.001 cold and hot immersion performance. (Figures 2&3).
Figure 2: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on tail flick latencies (46oC) in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, *** P<0.001 Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.

Figure 3: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on tail flick latencies (4oC) in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, *** P<0.001 Vs diabetic control group. One Way ANOVA followed by Bonferroni multiple comparisons.
Measurement of Muscle Incoordination using Rota Rod
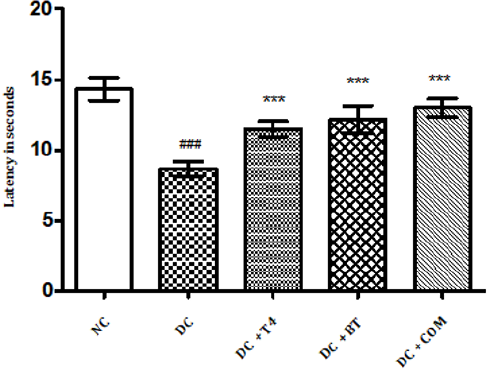
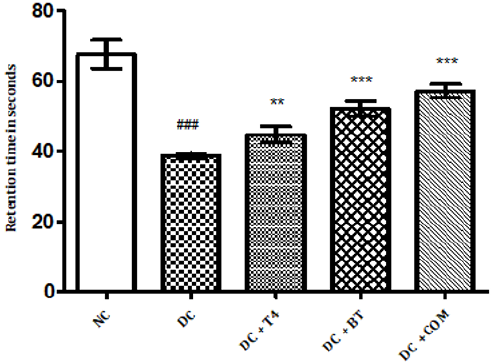
Time latencies at both 15, 25 rpm of normal rats was found to be 103.8±1.74,67.67±1.687 respectively, time latencies of diabetic rats at both 15,25 rpm was found to 75.5±1.176 38.83±0.307 and same were significantly reduced (P<0.001) as compared to normal control. Time latencies of diabetic rats treated with T4, BT and Combination at both 15, 25rpm was found to be 86.67±0.7149, 44.83±0.477, 86.83±0.792, 52.17±0.872, 91.5±0.846, 57.17±0.792 respectively and same were significantly P<0.001 improved (Figures 4&5).
Figure 4: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on muscle incordination by rota rod performance (15rpm) in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are epresented as mean ± SEM (n=6). ### P<0.001 Vs normal control group *** P<0.001 Vs diabetic control group. One Way ANOVA followed by Bonferroni multiple comparisons.
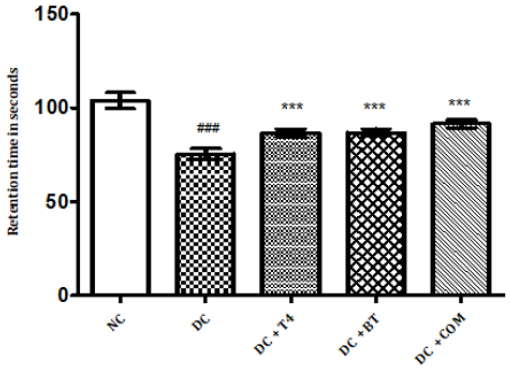
Figure 5: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on muscle incoordination by rota rod performance (25rpm) in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, **P<0.01, and *** P<0.001 Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.
Measurement of Grip Strength
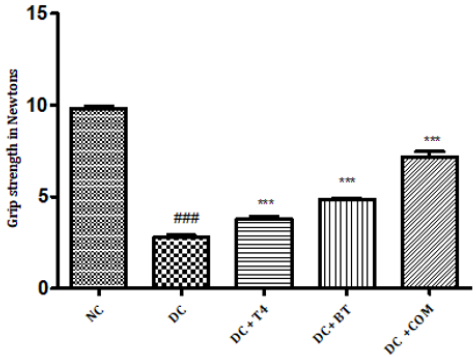
The grip strength of normal rats was found to be 9.822±0.1332,27.97±0.1171 respectively, The grip strength of diabetic rats was found to be 75.5±1.176, 38.83±0.307 and same were significantly reduced (P<0.001) as compared to normal control. Time latencies of diabetic rats treated with T4, BT and combination at both 15,25 rpm was found to be 86.67±0.7149, 44.83±0.477,86.83±0.792,52.17±0.872,91.5±0.846,57.17±0.792 respectively and same were significantly P<0.001 improved (Figure 6).
Figure 6: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on grip strength in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, *** P<0.001 Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.
Biochemical Studies
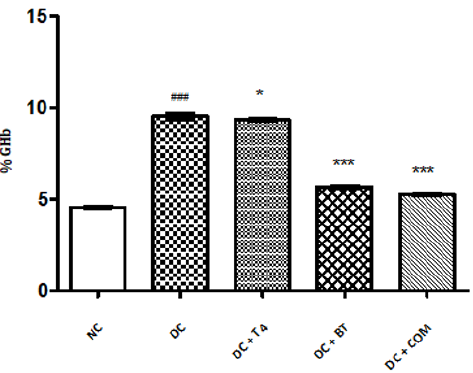
Estimation of GHb: The percentage of GHb of normal rats was found to be 4.577±0.0249, The percentage of GHb of diabetic rats was found to be 9.537±0.066, and same was significantly reduced ( P<0.001) as compared to normal control. The percentage of GHb of diabetic rats treated with T4, BT and combination was found to be 9.357±0.01838, 5.698±0.02561, 5.277±0.0261, respectively and same were significantly improved. P<0.05 when compared T4 treated diabetic rats with diabetic control. P<0.001 when compared BT, combination treated diabetic rats with diabetic control (Figure 7).
Figure 7: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on % GHb in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group,* P<0.05, and *** P<0.001 Vs diabetic control group.
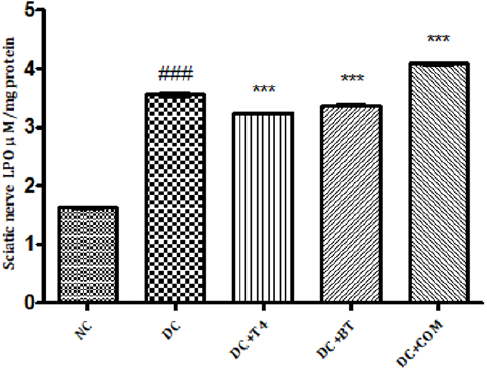
Measurement of Lipid Peroxidation: The sciatic nerve MDA levels of normal rats was found to 1.627±0.008433, The sciatic nerve of MDA levels diabetic rats was found to be significantly high (P<0.001) i.e 3.553±0.02860, The sciatic nerve MDA levels of diabetic rats treated with T4, BT and combination was found to be 3.235±0.008466, 3.368±0.009098, 4.080±0.01807, respectively and same were significantly (P<0.001) reduced (Figure 8).
Figure 8: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on sciatic nerve LPO in diabetic rats. NC: normal control, DC: diabetic control, COM: combination of thyroxine and benfotiamineValues are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, ***P<0.001, Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.
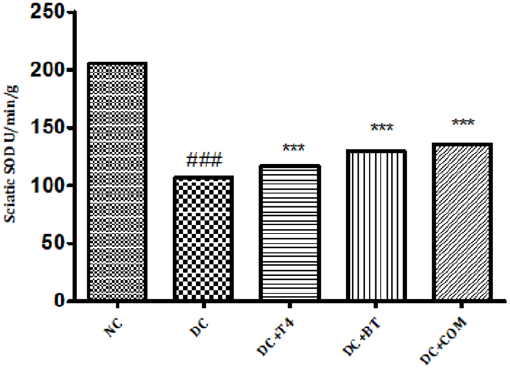
Estimation of Superoxide Dismutase (SOD): Sciatic nerve SOD activity in normal rats was found to be 205.7±0.1078, Sciatic nerve SOD activity was significantly (P.001) low 106.8±0.2798 in 5 weeks diabetic rats. SOD activity of diabetic rats treated with T4, BT and combination were found to be 130.0±0.2540, 135.6±0.1474 and 128.5±3.212 treatment significantly (P<0.001) restored SOD activity when compared to diabetic control (Figure 9).
Figure 9: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on sciatic nerve SOD in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, ***P<0.001, Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.
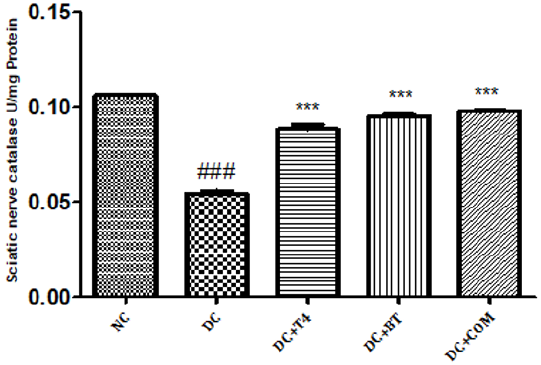
Estimation of Catalase: Sciatic nerve catalase activity in normal rats was found to be 0.1058± 0.0004, Sciatic nerve catalase activity was significantly (P.001) low 0.0545±0.0013 in 5 weeks diabetic rats. Catalase activity of diabetic rats treated with T4, BT and combination were found to be 0.08833±0.00230, 0.0950±0.00096and 128.5±0.00047 treatment significantly (P<0.001) restored catalase activity when compared to diabetic control (Figure 10).
Figure 10: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on sciatic nerve catalase in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, ***P<0.001, Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.
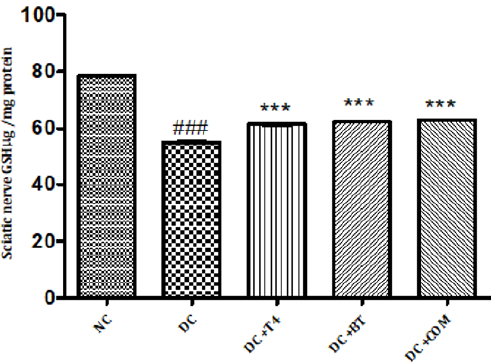
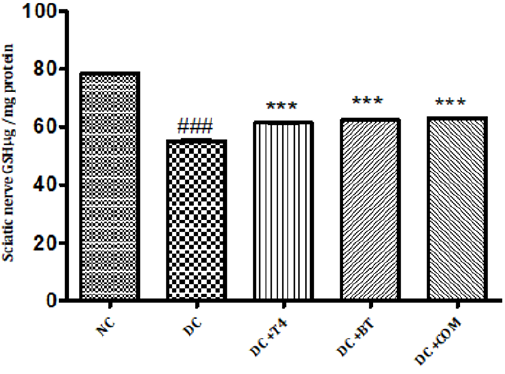
Measurement of Reduced Glutathione Activity: Sciatic nerve glutathione content in normal rats was found to be 0.1058± 0.0004, Sciatic nerve catalase activity was significantly (P.001) low 0.0545±0.0013 in 5 weeks diabetic rats. Catalase activity of diabetic rats treated with T4, BT and combination were found to be 0.08833±0.00230, 0.0950±0.00096and 128.5±0.00047 treatment significantly (P<0.001) restored catalase activity when compared to diabetic control. (Figure 11).
Figure 11: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on sciatic nerve GSH in diabetic rats. NC: normal control, DC: diabetic control, COM: combination of thyroxine, and Benfotiamine Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, ***P<0.001, Vs diabetic control group. One Way ANOVA followed by Bonferroni multiple comparisons.
Measurement of Nerve Conduction Velocity (NCV)
Sciatic nerve conduction velocity was significantly (<P0.001) was significantly reduced in 5 weeks diabetic rats 44.11± 0.2907 when compared to normal 53.13±0.4599, T4 treated diabetic rats significantly (P<0.01) exhibited improved NCV 45.72±0.1954. BT and combination both have also significantly improved (P<0.001) NCV 48.01±0.1954, 48.64±0.1876 (Figure 12).
Figure 12: Effect of treatment of thyroxine, benfotiamine and their combination for five weeks on nerve conduction velocity in diabetic rats. NC: normal control, DC: diabetic control, T4: thyroxine, BT: benfotiamine COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, **P<0.01, and P<0.001 Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.
In Vitro Glycation of Proteins
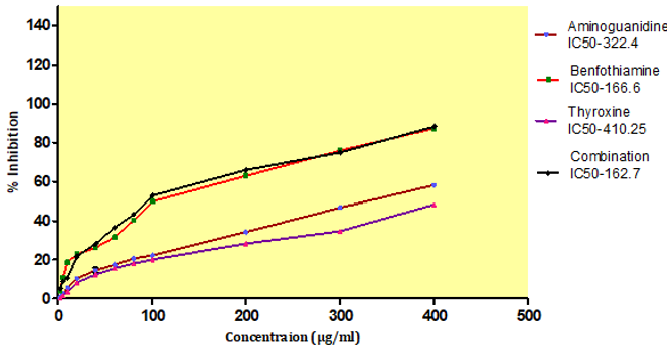
AGEs formation after incubation at 37ºC for 15 days, with an IC50 value of BT, T4, combination and standard aminoguanidine was found out to be 166.6±0.45μg/ml,410.25±0.32μg/ml, 162.7±0.37 μg/ml, 322.4± 2.23 μg/ml respectively. The combination exhibited higher inhibitory activity i.e.162.7±0.37μg/ml against AGEs formation after incubation compare to aminoguanidine (Figure 13).
Figure 13: Effect of thyroxine, benfotiamine and their combination on in-vitro AGE inhibition. NC: normal control, DC: diabetic control, COM: combination of thyroxine and benfotiamine. Values are represented as mean ± SEM (n=6). ### P<0.001 Vs normal control group, **P<0.01, and *** P<0.001 Vs diabetic control group. One Way ANOVA followed byBonferroni multiple comparisons.
Discussion
The major findings of the present study were that STZ induced diabetic rats showed significant weight loss, muscle incoordination, thermal and cold hyperalgesia, decreased grip strength, increased % GHb, electrophysiological abnormalities like decreased NCV and histological abnormalities when compared to normal rats. Treatment of diabetic rats with T4, BT and combination significantly improved the diabetes induced above deficits. Our results indicated that rats with diabetes induced by STZ showed body weight reduction during the experimental period. T4, BT and combination improved body weight from the initial value. Rats treated with combination exhibited less percentage of loss of body weight compared to T4 and BT alone thus the combination improved general health of rats by improving the body weight. DPN is associated with neuropathic pain which is most common in DPN, thus we evaluated the nociception in diabetic rats. Nociception was evaluated by thermal, cold hyperalgesia and was well evident in diabetic rats, which is in accordance with several other reports [39,37]. In the present study a significant reduction in nociception with T4, BT and combination treatment for five weeks was observed. The combination of T4, and BT exhibited synergistic effect on reducing nociception. The effectiveness of T4, BT and combination in neuropathic pain is further assessed by measurement of grip strength and muscle incoordination by grip strength meter and rotarod apparatus. Rotarod test was performed to examine the motor incoordination [40,38]. The Rotarod experiment demonstrated the impairment of the motor function and coordination in the diabetic rats. Diabetic rats showed shorter fall off time from the rotating rod when compared to control, suggesting impairment in their ability to integrate sensory inputs with appropriate motor commands to balance their posture. The T4, BT and combination treated diabetic rats increased the fall off time from the rotating rod compared to STZ-induced diabetic rats. Our results showed that T4, BT and combination normalize the motor function and coordination thus helps to maintain their posture during movement on the rod. The severity of diabetic neuropathy has been associated with decreased muscle strength in both type 1 and type 2 diabetes [41,39]. In our study we observed significant improvement in motor behavior particularly grip strength in addition to motor incordination after treatment with T4, BT and combination. The combination of T4, and BT thus exhibited synergistic effect on muscle incordination and gripstrength Marked increase inpercentage of Glycosylated haemoglobin (GHb) has been reported in previous studies in diabetic rats [42,40].
The levels of GHb is the marker of state of diabetes over a period of 90 days. Similar observations were found in diabetic control rats. In our study T4, BT and combination treated diabetic rats showed decreased HbA1levles. The decrease levels of HbA1 in T4 treated ratscould be due to decreasing elevated glucose by promoting cell differentiation into insulin-producing β-cells, upregulation of insulin, glucose transporter protein-2 transcripts, and insulin release [19], thus less glucose available for glycation with hemoglobin. The inhibition activity of T4 on glycation of proteins In Vitro was also measured in our study. T4 inhibited AGE formation In Vitro could be the contributing factor in inhibition of GHb in diabetic rats. Similar trend was observed in BT and combination treated rats. BT is a transketolase (TK) activator [43,41]. BT was shown to prevent experimental diabetic retinopathy and In Vitro hyperglycemia-induced endothelial dysfunction. The effects of benfotiamine on in vivo endothelial function remained unknown. BT has shown to inhibit hexosamine pathway, advanced glycation end product (AGE) formation pathway and the diacylglycerol (DAG) protein kinase C (PKC) pathways. BT was significantly decreased levels of HbA1 as discussed earlier could be due to inhibition of AGE formation as it was evidence by inhibition of In Vitro AGE formation in our study. The protective role of BT also probably due to its activity as co enzyme in various biological pathways [44,42]. The combination of BT and T4 has also shown synergistic effect in decreasing GHb.
A number of reports indicate that DPN is a hypoxic neuropathy. Decreased nerve blood flow may lead to decreased nerve conduction velocity (NCV) due to lower Na+-K+-exchanging ATPase activity [4]. Reactive oxygen species (ROS) such as superoxides and hydroxyl radicals cause vascular endothelial damage and reduced nitric oxide mediated vasodilatation. Studies have also showed evidence that superoxides and proximitized impairs endothelium dependent vascular relaxation of epineural arterioles of the sciatic nerve in diabetic rats [45,43]. In addition to vascular mechanisms nonvascular mechanisms like impairment of neurotrophic support have also been reported to cause nerve conduction deficits in DPN [46,44]. Enhancement of neurotrophic factors by prosaposinderived peptide was reported to preserve nerve conduction [47,45].The deficit in nerve conduction velocity was completely prevented by T4 treatment could be due to increased Na+-K+- exchanging ATPase activity, providing neurotrophic support and improving micro vascular circulation [48,46] further, T4 improved the endogenous antioxidants and decreased LPO in sciatic nerve homogenate. BT, combination treated rats were also exhibited augmented NCV could be due to the ability of BT to inhibit AGE formation, improved endogenous sciatic nerve antioxidant thus inhibiting AGE, free radicals induced nerve damage in our study. Thus these two vascular and nonvascular effects of BT and T4 would be the contributing factors for the synergistic activity in combination treated rats Morphological study of siatic nerve of normal rats showed closely packed nerve fibers normal endoneuria matrix separating the nerve fibers (Figure 14A), admixture of large and small diameter myelinated fibers and the thickness of the myelin sheath is proportionate the width of the axonal diameter (Figure 14 B). Conversly, diabetic rats displayed the histological damages like reduced nerve fiber density in the endoneurium, (loss of more of small myelinated fibers while large diameter fibers are better preserved), (endoneurial (Figure 15A), vascular thickening (diabetic microangiopathy) (Figure 15 B), However, myelin sheath was unaffected (Figure 14 B) by streptozotocin in DPN [49,47]. The altered sodium cell gradient due to impairment of Na+/K+ ATPase activity leading to altered membrane environment which in turn causes histological damages, and decrease myelin protein expression.
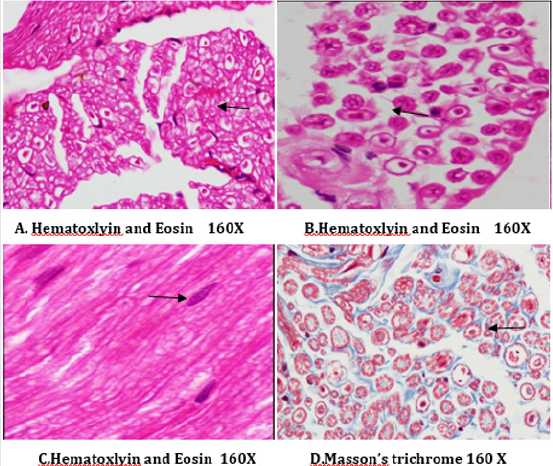
Figure 14: Histology of rat sciatic nerve of normal control group: (A) light microscopy transverse section showing closely packed nerve fibers and an occasional endoneurial blood vessel. (B) light microscopy transverse section showing individual nerve fibers and a central axon surrounded by a sheath of myelin.(C) longitudinal section showing the elongated schwann cell nuclei and longitudinally oriented axons with myelin sheaths.(D)transverse section of special stain for collagen highlights the endoneurial matrix separating the nerve fibers and collagenous component is stained blue.
T4, BT and combination treated rats sections showed absence of diabetic vascular changes, no vascular thickening to mild vascular thickening (Figure 15B), normal density of fibers with preservation of small and large diameter fibers, presence of regenerating nerve clusters (Figures 16-18 ).Treatment with T4, BT and combination for five weeks almost completely prevented the histological damages induced by diabetes. T4, BT could probably prevent the histological damages induced by diabetes due to prevention of hyperglycemia induced vascular and non-vascular mechanisms.
Figure 15: Histology of rat sciatic nerve of STZ (Diabetes) control group. (A)light microscopy of transverse section showing reduced nerve fiber density in the endoneurium.(B) transverse section of special stain for myelin reveals more of small myelinated fibers affected while large diameter fibers are better preserved, myelin sheaths are of normal thickness.(C) transverse section show diabetic microangipathic changes with endoneurial vessel wall thickening and collagenous hyalinizationhighlighted by the masson’s trichrome stain (D).
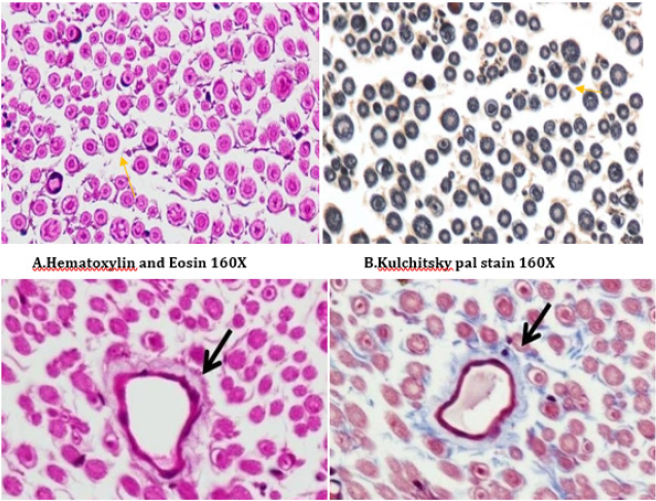
Figure 16: Histology of rat sciatic nerve of STZ (Diabetes) control treated with thyroxine. (A)light microscopy of transverse section showing preserved fiber density. (B) transverse section showing absence of diabetic vascular changes no vascular thickening to mild vascular thickening (arrow). (C) transverse section of special stain for myelin show near normal density of fibers with preservation of small and large diameter fibers, presence of regenerating nerve clusters.
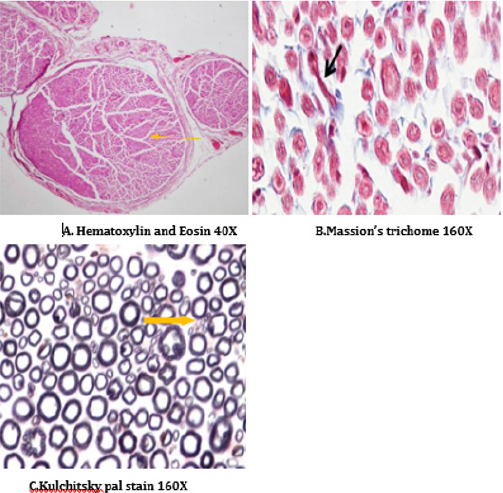
Figure 17: Histology of rat sciatic nerve of STZ (Diabetes) control treated with benfotiamine. (A)light microscopy of transverse section showing normal density of nerve fibers. (B) transverse section showing no vascular thickening.(C) transverse section of special stain for myelin show normal density of small and large diameter fibers, the myelin sheaths are stained dark.
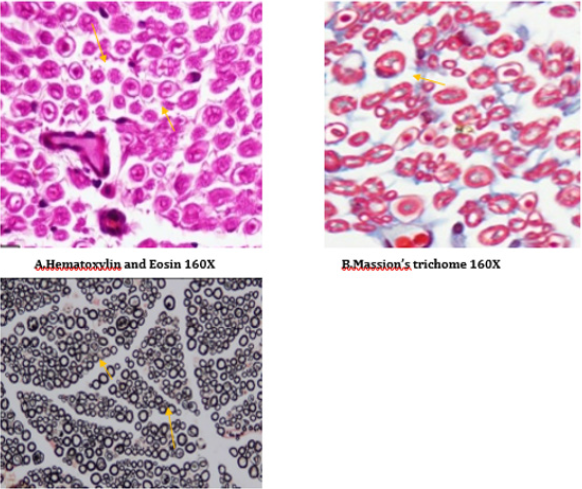
Figure 18: Histology of rat sciatic nerve of STZ (Diabetes) control treated with combination of thyroxine and benfotiamine. (A) light microscopy of transverse section showing sparse endoneurial interstitial inflammation in one case. (B) transverse section showing no endoneurial vascular thickening (C) transverse section of special stain for myelin show preserved density with presence of large and small diameter fibers.
Conclusion
Treatment with T4, BT, and combination effectively prevented many of the behavioural, electrophysiological and histological manifestations of diabetes induced peripheral neuropathy by decreasing thermal and cold hyperalgesia, improving motor incoordination, grip strength, NCV, fiber density.
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online

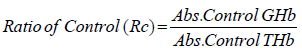





No comments:
Post a Comment