Lupine Publishers| Journal of Diabetes and Obesity
Abstract
Background: Metabolic Syndrome (MS): Overweight, Obesity, Hypertension, Hyperglycemia and Hypercholesterolemia, are generally accepted today as clinical signals leading to cardiovascular diseases. Control of MS is therefore of a common health concern. While drug treatment is yet not available or may not be creditable, developing an effective health supplement against MS is highly justified.
Methods: A herbal formula composed of four herbs known to have anti MS pathological effects was used for in vivo and in vitro biological researches to verify its pharmacological effect, and subsequent pilot clinical trial.
Results: In vitro study: Adipocyte viability and cholesterol uptake, liver cell viability and anti-glycaemia effects, all gave positive results of good control. In vivo study testing herbal formula’s effects on obese mice also showed very promising results. In clinical trial, measurements of body weight, body circumferences, BMI, as well as liver fibrosis, all showed good responses after the herbal formula consumption.
Conclusion: Our efforts on the creation of an Evidence-Based Specific Supplement for the control of Metabolic Syndrome have harvested highly positive data in the laboratory. The subsequent 3 months’ pilot clinical trial showed positive data on the control of blood lipids, general body measurements and liver steatosis.
Keywords: Gene Expression; Lipid Droplets; Mitochondria; RNA Sequencing; Type 2 Diabetes Mellitus
Introduction
As cardiovascular diseases have become dominant causes of
mortality, other related pathological presentations are gaining
public attention [1,2]. Obesity is the commonest observable
indicative of “unhealthiness”, leading to cardiovascular problems.
Thus, simple objective measurements of obesity like Body Mass
Index (BMI), blood lipids and cholesterols are also gaining much
public concern [3]. Blood pressure and blood sugar levels naturally
fall into the same checklist of safety requirements which are mainly
affecting cardiovascular health [4].
As time goes on, the five major influences on cardiovascular
health have thus been put together as the “Metabolic Syndrome”
(MS): overweight, obesity, hypertension, hyperglycemia and
hypercholesterolemia [5]. Another associated pathology, viz. fatty
liver, turns up, as many sufferers of MS are found to have different
degrees of liver dysfunction and fat deposits gradually leading the
way to liver fibrosis [6].
For decades, varieties of interventions are available for
the control of the different aspects of MS, particularly in areas that require specific effective control like hypertension and
hyperglycemia. It is realized now that a better way to deal with the
problem is to broaden the area of concern so that MS can be taken
together in a multi-facetted preventive endeavor. Although specific
single target pharmaceuticals are available for solitary treatment, a
more ideal alternative could be some extra-pharmaceutical multiple
target, harmonizing supplement to take care of all components of
the MS [7,8].
Medicinal Herbs
A number of Medicinal herbs have been traditionally used as
supplement for patients with diabetes mellitus and obesity. They
are chosen for our study on metabolic syndrome. Platform studies
would include bioactivities related to cardiovascular problems
[9,10].
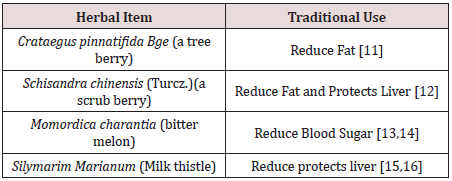
We selected four edible herbals items for the Chinese
pharmacopeia to be tested. They are: (Table 1).
The combined formula (2MSC) would be tested for the control of body fat and sugar metabolism, while its effects on blood pressure, blood lipids and liver function would carefully be studied [17]. The design of this study on MS has chosen an emphasis on the effects on fatty liver [18].
Methods
Preclinical Studies
In vitro experiments included the following:
To investigate specific bioactivities of individual herbs and the combined formula
I.Testing the effects of Crataegus Fructus and the formula on the adipocyte viability and cholesterol uptake using adipocyte and cacocell cultures [19].
II.Testing the effects of Schisandra Fructus and Silymarim Marianum separately and the combined formula on liver cell viability using HepG2 cells [20].
III.Testing the antiglycemic effects of Momordica and the combined formula using Brush Border Membrance Vesicle model via its glucose uptake obstruction [21-23].
In vivo Experiments
Male C578 1/6 mice were use. Obesity induction was achieved using forced high-fat feeding for 8 weeks. The obese mice were treated with normal diet and continual high fat diet with 2% and 4% combined herbal formula [18]. At the end of 8 weeks for the low dose and 12 weeks for the high dose, the animals were sacrificed to have comprehensive checks on Body Weight, Blood examinations for lipid assessments and liver examinations.
Results of Laboratory Studies
In Vitro Studies
3T3-L1 preadipocytes differentiation cell assay showed the
effects of the different concentrations of 125 μg/ml, it was a dose
that induced significant toxicity to cells, and there was no dose–
response effect observed [19].
Fluorescent tagged cholesterol-treated Caco-2 cell assay
The effect of the different concentrations of Crataegus Fructus aqueous extract and the herbal formula extract on cholesterol uptake in Caco-2 cells. Crataegus Fructus aqueous extract significantly prevented the cholesterol uptake in Caco-2 cells in a dose dependent manner. Herbal formula extract on the other hand had no significant effect on the cholesterol uptake in Caco-2 cells [19].
Animal Studies
The body weight, adipose tissues weight and liver weight were
measured. In the 8-week treatment study, the High-fat diet (HF-fed)
animals significantly gained more weight than chow-fed animals.
Among all HF-fed animals, there was a trend for a reduction in body
weight of 4% among herbal formula fed animals, starting from
week 9 onwards. Three types of adipose tissues (epididymal fat,
peri-renal fat, and inguinal subcutaneous fat) were isolated and
weighed. High-fat diet induced obesity in mice compared to normal
chow-fed mice after 16 weeks and 20 weeks, as evidenced by the
significant increase in all three types of fat pad mass to body weight
ratio: epididymal fat pad (p < 0.01 for both 8-week and 12-week
treatment studies), inguinal fat pad (p < 0.01 for both 8-week and
12-week treatment studies), and perirenal fat pad (p < 0.001 for
both 8-week and 12-week treatment studies).
Livers were isolated, weighed and presented as liver to body
weight ratio. High-fat diet induced an increase in liver to body
weight ratio in both 8-week and 12-week treatment studies [19].
2% and 4% herbal extracts dose-dependently reduced the liver to
body weight ratio in both treatment periods.
Liver histopathology and inflammation assessment
Livers from mice fed on different diets were analyzed histologically. Normal-chow-fed animals demonstrated the histological sections of normal livers. In contrast, H&E sections from HF animals revealed the presence of a large number of circular lipid droplets within the hepatocytes. These lipid inclusions were clearly reduced in size as well as number in the livers of all herbal formula treated animals.
A Pilot Clinical Trial
Title: Pilot Clinical Study to evaluate the effects of the innovative herbal formula 2MSC in subjects with Metabolic Syndrome.
Hypothesis: 2 MSC is effective for the Management of MS, with particular emphasis on liver fibrosis (Fatty liver).
Study Design
An open label pilot study conducted with 30 overweight adult men and women were assigned to take 2 MSC daily for 3 months.
Study Population
Subjects recruited were 40-66 years of age, with BMI between ≥25kg/m2 and ≤37.7kg/m2. They agreed to attend all study visits and to keep their normal dietary habits and usual physical activities. Subjects were excluded if they were diabetic (on diabetic medication for more than four weeks); on cardiac and statin related drugs; on immune-suppressive drugs. Cigarette smokers, pregnant or lactating women were excluded. A total of 30 volunteers with no past history of allergy to herbal medicine were recruited.
Study Procedures
The study started after signing the proper consent. Duration of treatment lasted 12 weeks. Monthly phone calls were conducted to monitor the progress, compliance and adverse effects. Volunteers were reminded to keep their usual dietary habits and physical activities.
Data Collections
Demographic and basic measurements related to MS including Body Weight, BMI; Waist Circumference; Hip Circumference and Neck Circumference. Blood testing included fasting blood sugar, Liver function tests, Renal function tests. Other items related to Lipid metabolism included total cholesterol (TC), Triglycerides (TG), Low density (LDL) and high-density lipoprotein cholesterol (HDL), and non-high-density lipoprotein cholesterol. Adiponectin and some immune cytokines were also taken. Fibroscan of the liver was done at baseline and final visit. The Quality of life was checked with the standard SF-36 scoring sheet.
Primary Outcome
The primary outcome included the decline of Body Weight, Waist Circumference and Lowing of blood triglyceride TG.
Statistical Analysis
Excel 2016 (Microsoft Corp, Redmond WA) was used for data entry. Statistical analysis was performed using SPSS Base System ver. 25 (IBM SPSS Inc., Chicago IL.). Statistical analysis (descriptive statistics and Student t tests) was performed using SPSS ver. 22 (IBM SPSS Inc., Chicago IL.). Paired t-test was utilized to evaluate the difference between pretreatment mean and post-treatment mean. The percent changes in CAP Reading from baseline to 12 weeks of treatment with 2MSC were analyzed by using Chi-Square test.
Results
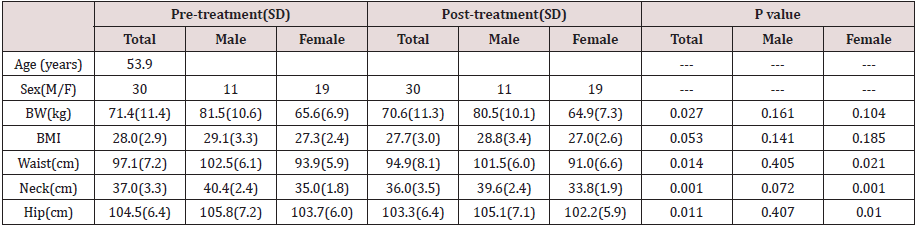
Compliance was excellent. No subject withdrew during study period. Adverse effects reported were all mild, including loose stools and mild abdominal discomfort. Liver and kidney functions remained normal. Bodily measurements all showed clear tendencies of improvement with convincing p values (Table 2).
The blood checking data indicative of lipid metabolism
also showed very positive decline in the triglycerides (Table 3).
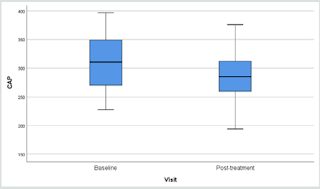
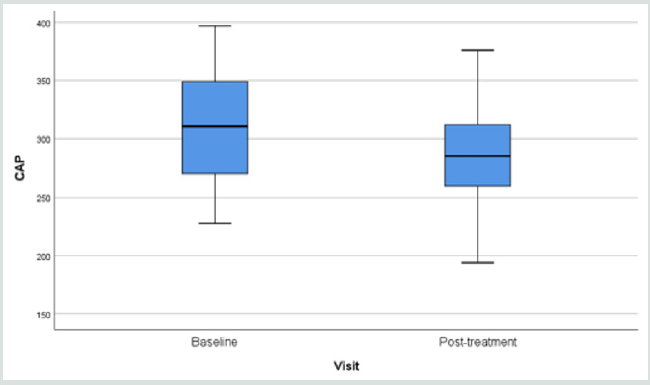
Fibroscan study showing the “rigidity” of the liver through the
anterior abdominal wall, demonstrated softening from 315.3 (49.7)
to 291.0 (44.1) p=0.006 (Table 3).
Results of fibroscan for fatty liver study gave an overall positive
effects of the herbal formula slowing down the progress of liver
fibrosis when the controlled attenuation parameter (CAP) data
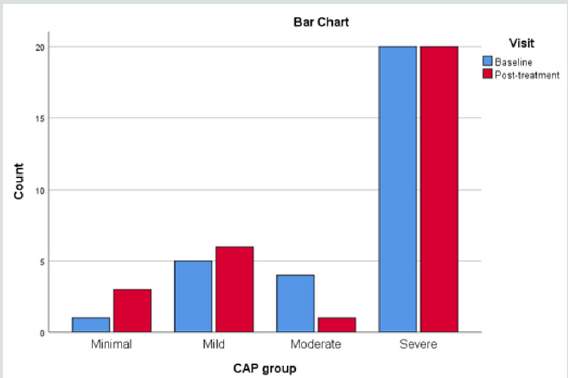
revealed by the fibroscan were analyzed (Figure 1).
Table 3: Changes in the lipid profile and Fibroscan after administration of 2MSC.
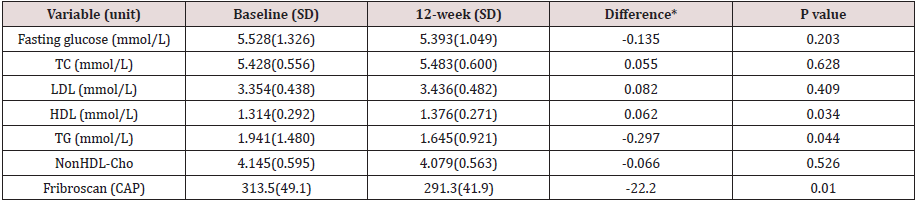
TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; * 12-week minus Baseline.
When the liver conditions of the volunteers were classified into four different groups of liver steatosis, as: minimal, mild, moderate and severe, it was interesting to find that the herbal formula influenced only the moderate group significantly, while the minimal to mild group and severe group were unaffected. (Figure 2).
Discussion
Functional foods or nutraceuticals which have potential anti-obesity properties have attracted great attention. Schisandrae Fructus is a Chinese herb traditionally used as a liver tonic. Silymarin, an extract of the milk thistle (Silybum marianum), is a dietary supplement that is widely used in Europe for the prevention and treatment of liver problems. Crataegus Fructus (hawthorn) is traditionally used to promote digestion and dissipate food stagnation. Momordica charantia (bitter melon) is traditionally used for the treatment of diabetes in Ayurvedic Medicine. Our in vitro results suggested Crataegus Fructus aqueous extract exerted potent inhibitory effects on 3T3-L1 preadipocytes differentiation and cholesterol uptake into Caco-2 cells. Schisandrae Fructus aqueous extract and milk thistle exerted inhibitory effects on oleic acid-induced fatty liver in HepG2 cells. Momordica charantia extract on the other hand, exerted significant inhibitory effects on the glucose uptake into BBMV. Our in vivo results showed that our herbal formula exhibited a trend to reduce diet-induced increase in body weight and fat pad mass (epididymal, perirenal and inguinal fat). It also significantly reduced diet-induced increase in liver weight, liver lipid, and plasma lipid dose-dependently. High-fat diet induced a significant reduction in adiponectin level which was significantly improved by herbal formula supplementation at 4%. The herbal formula also significantly reduced diet-induced inflammation in the liver at both doses.
Metabolic Syndrome is currently understood as a combination
of commonly co-existing symptoms some of them clearly present
uniquely as an outstanding disease, like diabetes mellitus and
hypertension, while others present as potentially threatening
pathologies. Although adequate treatment for diabetes and
hypertension are available, there is a common wish that they could
be controlled at an early stage, so that progression could be curbed
[23].
Apart from a rigidly followed lifestyle recommended for the
cardiovascular system and obesity, which is not easy for the general
public, food supplements have been used to help [24,25]. Our pilot
clinical study ventured to develop an evidence-based supplement
specific for MS, adopting a broad view covering all five problems:
obesity, body weight, BMI, blood pressure, and blood lipids.
Changes in liver fat and adiponectin were also explored [26,27].
Our pilot clinical study showed an overall body weight loss from
71.4 to 70.6 kg (p=0.027) and a BMI 28.0 to 27.7 (p=0.053) within
a short period of 12 weeks.
The body circumference (external fat collection) shrinkage as
detected through waist and hip also showed significant decline
(p=0.014 and 0.011).
Since neck circumference measurement has recently been
endorsed as a simple practical assessment for body fat, our neck
measurements were supportive to the innovation and showed a
37.0 to 36.0 cm decline [28,29].
Useful results were provided in the blood tests. Liver function
and renal function tests stayed normal, indicating the safety of
the formula. With regard to the lipids: there were declines in
triglycerages (p=0.044) and an increase in high density lipid
(p=0.034) [30]. Fasting blood sugar remained stable.
The overall results were promising but the trial period lasted
only 12 weeks and the number of volunteers was small. More than 50
Traditional Chinese Medicine formulae have been used to treat MS,
basing on classical theory “Kidney Health”, cardiovascular support,
and general harmonizing effects [31]. The 4-herbs selected to form
the 2MSC formula were taken from classical recommendations
subsequently scrutinized on bioactivity platform, to provide
pharmacological evidence.
Liver scanning using Ultrasonic Fibroscan device is a simple
qualitative and quantitative way to evaluate fat contents in the
liver suspected of fatty changes. In our study, liver tissue resistance
to Ultrasonic pressure was found to decline from 315.3 to 291.3
(p=0.010) within a period of only 12 weeks (Figure 1) (32-35).
There are many reports on the study of non-alcoholic fatty liver
in response to supplements [25,36-39] mainly targeting on the
liver pathology alone. Our study took a broader view: other factors
leading to MS should also be contributing towards fatty changes in
the liver.
Since the discovery of Adiponectin as a factor very much affecting
the Metabolism of fatty tissues responsible for the metabolic cycle
connecting fat deposit and carbohydrate consumption, the behavior
of adiponectin is often included in studies related to MS [40-43].
In our preclinical animal experimental study lasting 12 weeks, we
found a high dose of the combined formula gave an increase of
adiponectin suggesting that carbohydrates metabolism could have
been promoted [19]. In our pilot clinical trial, however adiponectin
was found to be either raised, remained stable or decreased. This
might be due to the low dose effect or that the study lasting only
12 weeks. Using the SF36 questionnaire for the volunteers’ selfevaluation
of their physical, mental and social well-being’s did not
give remarkable results.
Conclusion
Up to today there is no FDA approved medications for the treatment of non-alcoholic fatty liver disease (NAFLD). The American Association for the Study of Liver Diseases (AASLD) suggested the combination use of vitamin E (an antioxidant) and pioglitazone may be helpful but not all patients would benefit from it. For patients diagnosed with NAFLD, the first line of treatment usually involves weight loss through a combination of a healthy diet and exercise. According to the AASLD guidelines, it was recommended that 10% body weight loss would lead to improvement of the steatosis and inflammation related. Previous studies also found that lifestyle modification could significantly improve the mean fibroscan CAP value (278.57±49.13 dB/m VS 252.91±62.02 dB/m, p=0.03). Thus 6 months of lifestyle modification which include moderate intensity physical exercise 3 days per week each for 45 minutes; plus a restricted caloric intake of 25-30 kcals/kg/day could better protect liver health after tremendous individual efforts and This obviously involves hard work and determination.
Our efforts on the creation of an Evidence-Based Specific Supplement for the control of Metabolic Syndrome have harvested highly positive data in the laboratory and in the subsequent 3 months’ pilot clinical trial. Encouraging results were obtained in the control of blood lipids, general body measurements and liver fibrosis. It is envisaged that coupled with more exercises and dietary control, better results could be expected. Further clinical studies would be very much warranted.