Lupine Publishers| Journal of Diabetes and Obesity
Abstract
Background: Once weekly (OW) semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) currently under evaluation for treatment of obesity at a dose of 2.4 mg OW.
Objective: To compare weight-loss efficacy and safety of once daily (OD) liraglutide 3.0 mg versus OW semaglutide 2.4 mg. Methods: Pubmed research up to March 31, 2021. Randomized trials, pertinent animal studies, and reviews are included. Search terms were glucagon-like peptide-1 receptor agonists, weight loss, obesity, liraglutide, semaglutide, efficacy, safety.
Results: No head to head trials are available to provide direct comparison of efficacy of OD liraglutide 3.0 mg versus OW semaglutide 2.4 mg. However, marked resemblance between trials in terms of study protocols and subjects’ characteristics may allow indirect comparison. In clinical trials of OW semaglutide, this drug was consistently associated with greater weight loss than in trials of OD liraglutide. Thus, placebo-corrected percentage weight reduction was -10.3 to -12.4% and -5.4% with OW semaglutide and OD liraglutide, respectively. In patients with type 2 diabetes, corresponding weight reduction was less pronounced with both drugs being -6.2% and -4.3% with OW semaglutide and OD liraglutide, respectively. In addition, head to head trials comparing liraglutide and semaglutide used in different doses and formulations consistently showed more weight loss in favor of semaglutide. In general, the anti-hyperglycemic efficacy and safety profile are similar in both drugs.
Conclusions: Available indirect evidence suggests that OW semaglutide 2.4 mg may be superior to OD liraglutide 3.0 mg for weight loss. Head-to-head comparison between these 2 agents is essential to confirm this conclusion.
Keywords: Obesity; Liraglutide; Semaglutide; Glucagon-like Peptide-1; Efficacy; Safety; Weight Loss; Type 2 Diabetes; Hemoglobin A1c
Introduction
GLP-1 RAs are approved for treatment of type 2 diabetes. The drug profile of these drugs is characterized by mild dose-related weight loss of approximately 2-6 kg [1]. Currently, liraglutide is the only GLP-1 RA approved for treatment of obesity in a dose higher than that approved for type 2 diabetes (3.0 mg daily for treatment of obesity as opposed to a maximum dose of 1.8 mg/d in type 2 diabetes) [2]. Semaglutide is another GLP-1 RA approved for treatment of type 2 diabetes in a dose of 0.5-1.0 mg given subcutaneously OW and as an oral formulation in a dose up to 14 mg once daily [3,4]. Currently, semaglutide is under evaluation for future approval for treatment of obesity. The Semaglutide Treatment Effect in People with obesity (STEP) development program including 5 phase 3 clinical trials (STEP 1 to 5) was launched to evaluate efficacy and safety of OW semaglutide at this high dose of 2.4 mg for treatment of obesity in patients with and without diabetes [5].
Mechanisms of Weight Loss by Liraglutide and Semaglutide
In general, the mechanisms of weight loss by liraglutide and semaglutide are similar. Both agents were shown to reduce appetite and hunger while increasing sense of fullness and satiety [6,7]. In addition, OW semaglutide 2.4 mg, but not liraglutide, may decrease food craving [7]. Animal studies have shown that the anorexigenic effect of semaglutide is mediated by GLP-1 receptors in the hypothalamus and hind brain [8,9]. Delay in gastric emptying, a class effect of all GLP-1 RAs, may contribute to the sensation of early fullness [10]. Meanwhile, one study with relatively longfollow- up (52 weeks) has shown that improvements in hunger and fullness with OD liraglutide 3.0 mg peak after 4 weeks, then decline gradually and return to baseline after 40 weeks [6]. Similar followup studies are not available for semaglutide.
STEP Program of Semaglutide
STEP 1 to 4 trials are well-designed studies comparing OW 2.4 mg semaglutide with placebo in obese individuals (defined as BMI of ≥ 30 kg/m2, over ≥ 27 kg/m2 with ≥ 1 weight-related coexisting condition e.g. hypertension, dyslipidemia, cardiovascular disease, or obstructive sleep apnea) for 68 week-duration [11-14]. STEP 1, 3 and 4 excluded patients with diabetes, whereas STEP 2 included exclusively patients with type 2 diabetes [11,13-14]. In addition, STEP 2 included a third group of individuals randomized to the smaller anti-diabetic dose of OW semaglutide 1.0 mg [12]. In STEP 1, 2 and 4, all participants receive lifestyle intervention defined as a 500 kcal deficit relative to the estimated energy expenditure plus encouragement of increase physical activity, such as walking 150 minutes per week. In STEP 3 trial, all subjects received a low-calorie diet (1000-1200 kcal/d) provided as meal replacement for the first 8 weeks. Subsequently, they were transitioned to a low-calorie diet (1200-1800 kcal/d) of conventional food. Moreover, they were prescribed 200 min of physical activity/week [13]. The coprimary endpoints of STEP 1 to 3 trials were the percentage change in body weight and weight reduction of at least 5% at week 68 compared with placebo [11-13]. STEP 4 trial was a withdrawal trial that includes an initial run-in period of 20 week during which all subjects received OW semaglutide 2.4 mg followed by randomization to a group that continued the drug and another group that switched to placebo for further 48 weeks [14]. Overview of STEP 1 to 4 trials are summarized in (Tables 1 and 2).
Table 1: Weight-loss efficacy of liraglutide and semaglutide in patients without diabetes.
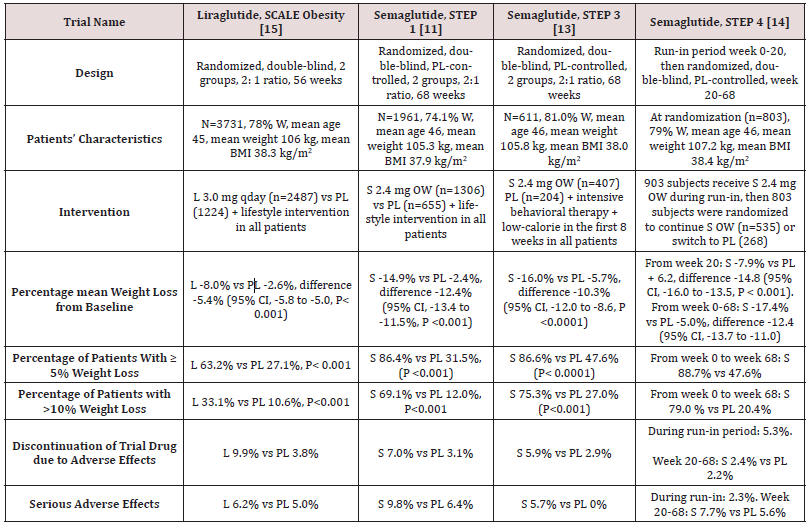
Abbreviations: W: Women; BMI: Body Mass Index; L: Liraglutide; S: Semaglutide; OW: Once Weekly; PL: Placebo; HbA1c: Hemoglobin A1c; CI: Confidence Intervals
Table 2: Weight-loss efficacy of liraglutide and semaglutide in patients with type 2 diabetes
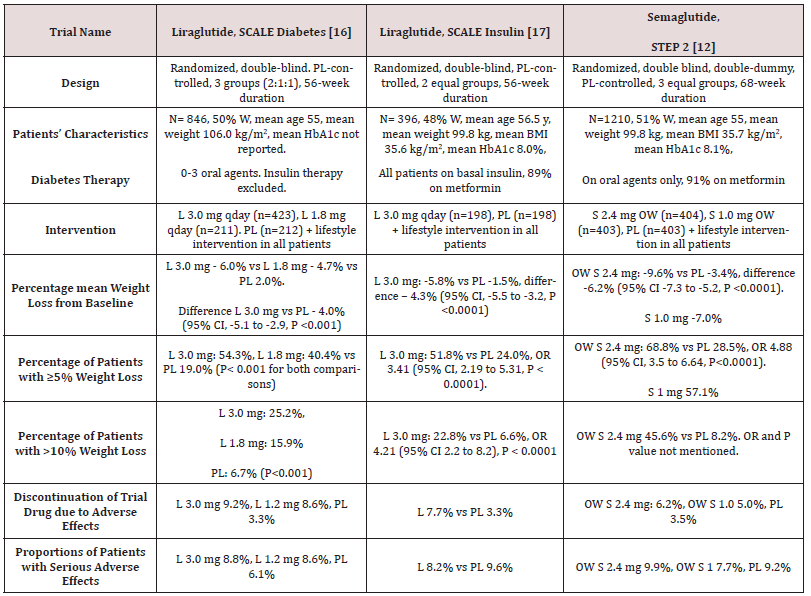
Abbreviations: PL: Placebo; W: Women; HbA1c: Hemoglobin A1c; L : Liraglutide; OWS: Once-Weekly Semaglutide
Weight loss in Semaglutide and Liraglutide Trials
While no head to head trials are available to compare weight loss efficacy of OW semaglutide 2.4 mg with OD liraglutide 3.0 mg, indirect comparison may be inferred from results of their respective trials. In fact, as shown in tables 1 and 2, subjects’ characteristics at baseline in these trials were similar to a great extent (Table 1). In addition, the study protocols and designs have several common features (e.g. similar primary end point). In STEP trials 1, 3 and 4 that excluded patients with diabetes, the difference in weight loss between OW semaglutide and placebo ranged between -10.3% and -12.4% at 68 weeks (Table 1). Meanwhile, in the SCALE Obesity and prediabetes trial of OD liraglutide 3.0 mg, the corresponding difference was -5.4% (95% CI, -5.8 to -5.0%) at 56 weeks (Table 1) [15]. In trials that exclusively recruited patients with type 2 diabetes, the weight loss efficacy of both drugs was diminished, but was still relatively greater in OW semaglutide 2.4 mg than with OD liraglutide 3.0 mg. Thus, in 2 liraglutide diabetes trials, the mean difference in weight loss between the drug and placebo was -4.0 and -4.3%, whereas the corresponding difference was -6.2% with OW semaglutide 2.4 mg (Table 2) [12,16-17]. The explanation of this finding is unclear but might be related to the coexistence of type 2 diabetes, relatively older patient population (mean age approximately 55 year-old in diabetes trials versus 45 year-old in trials excluding diabetes), or the lower baseline body weight (approximately 99.8 kg in diabetes trials versus approximately 105.5 kg in non-diabetes trials) (Tables 1-2) [12,16-17]. Other parameters that suggest superiority of OW semaglutide 2.4 mg over OD liraglutide 3.0 mg are the proportions of individuals losing ≥ 5% and > 10% of body weight. These proportions were always higher in trials of semaglutide than in those of liraglutide (Tables 1 and 2).
Head to Head Trials of Semaglutide Versus Liraglutide
Another indirect line of evidence suggesting greater efficacy of semaglutide compared to liraglutide may be derived from 3 randomized head to head trials comparing the 2 agents in different doses and formulations. A randomized, placebo-controlled, doubleblind trial [18] compared semaglutide in 5 daily subcutaneous doses (0.05, 0.1, 0.2, 0.3, and 0.4 mg) versus liraglutide 3.0 mg once daily on top of lifestyle changes in obese subjects without diabetes. After 52 weeks, mean weight reduction from baseline was significantly greater in patients randomized to semaglutide doses ≥ 0.2 mg daily being - 11.2 to -13.8% versus -7.8% in subjects randomized to liraglutide 3.0 mg daily [18]. The second trial including patients with type 2 diabetes [19] compared oral semaglutide (14 mg qday) with OD liraglutide 1.8 mg in doubleblind double-dummy fashion. After 26 weeks, oral semaglutide resulted in superior weight loss (-4.4 kg) compared with liraglutide (-3.1 kg), estimated difference -1.2 kg (95% CI, -1.9 to -0.6, P= 0.001) [19]. The third trial [20] compared OW semaglutide 1.0 mg with liraglutide 1.2 mg in patients with type 2 diabetes in an open-label design. After 30 weeks, mean weight loss was -5.8 kg and -1.9 kg, in the semaglutide and liraglutide groups, respectively; estimated treatment difference -3.8 kg (95% CI, -4.47 to -3.09, P<0.0001) [20]. Taken together, the results of the preceding 3 trials suggest higher efficacy of semaglutide than liraglutide irrespective of doses or drug formulation (i.e. subcutaneous or oral semaglutide).
Anti-Hyperglycemic Efficacy of Liraglutide Versus Semaglutide
The difference between semaglutide and liraglutide with respect to their anti-hyperglycemic efficacy is not as consistent as in their weight-loss effects. Thus, in the studies conducted by O’Neil et al, [18] and Pratley et al [19], semaglutide was similar to liraglutide in HbA1c reduction. Meanwhile, in the trial conducted by Capehorn et al, [20], OW semaglutide 1.0 mg was superior to liraglutide 1.2 mg qday; estimated treatment difference in HbA1c reduction was - 0.69% in favor of semaglutide. However, the latter trial is limited by its open-label design and using liraglutide in submaximal antidiabetic dose (1.2 mg instead of 1.8 mg) [20]. Therefore, while semaglutide may be more effective than liraglutide in causing weight loss, both GLP-1 RAs may be equally effective in terms of glycemic control.
Effects of Semaglutide and Liraglutide on Cardiovascular Variables
Significant reduction in systolic blood pressure (SBP) was recorded in subjects randomized to semaglutide in STEP 1-3 trials, approximately 4-5 mmHg lower than in individuals randomized to placebo [11-13]. Likewise, a significant reduction in DBP of approximately 2 mmHg was observed in STEP 1 and 3 trials [11,13]. Changes in lipid panel were generally mild. Thus, reduction in plasma triglycerides of 14-17% compared to placebo was the most consistent change in lipid panel. Minor reductions in concentrations of low-density lipoprotein-cholesterol (LDL-C) (by ≤7% vs placebo) and increase in high-density lipoprotein-cholesterol (HDL-C) levels (by <5% vs placebo) were also observed. In addition, there was significant reduction in the inflammatory marker C-reactive protein (CRP) levels in semaglutide-treated subjects vs placebo [11-13]. Similar beneficial changes in the above cardiovascular (CV) markers were described in liraglutide trials albeit they were lesser in magnitude [15,21]. The above favorable changes in blood pressure, lipids and CRP are likely attributed to weight loss per se and are unlikely to be direct effects of semaglutide or liraglutide.
Safety of Liraglutide and Semaglutide as Anti-Obesity Agents
Gastrointestinal Adverse Effects
Gastrointestinal (GI) adverse effects represent the most common adverse events that characterize all GLP-1 RAs. In liraglutide obesity trials, GI adverse events occurred in 65% and 39% of subjects randomized to OD liraglutide 3.0 mg and placebo, respectively [16]. In STEP 1-3 trials of semaglutide, GI adverse effects were reported by approximately 63-83% and 34-63% in subjects randomized to OW semaglutide and placebo, respectively [11-13]. Among the GI adverse effects, nausea was the most common, followed by diarrhea, vomiting and constipation [11-13,16]. The frequency of GI symptoms increased early in the first few weeks during drug titration. They were generally described as mild to moderate and transient. However, in a minority of patients, they can be severe. In fact, GI adverse effects were the most frequent cause of premature drug withdrawal. Thus, in the largest obesity trial of liraglutide, drug discontinuation due to GI adverse effects occurred in 6.4% and 0.7% in the liraglutide and placebo group, respectively [15]. In STEP trials, withdrawal due GI adverse events occurred in 3.4-4.5% and 0-1.0% in patients randomized to OW semaglutide and placebo, respectively [11-13]. Previous trials including patients with type 2 diabetes using OW 1.0 mg semaglutide have shown that GI adverse effects tend to be more common with semaglutide compared with other GLP-1 RAs [1]. Meanwhile, post-hoc analysis by Lingway et al [1] suggest that GI adverse effects contribute minimally (less than 0.1 kg) to the superior weight loss effects of semaglutide vs other GLP-1RAs. Incidence of cholelithiasis and cholecystitis was slightly higher with liraglutide than placebo, 1.5% and 0.4%, respectively [15] as well as with semaglutide than with placebo, 2.5-2.6% versus 0-1.2% [11-13]. These events may be attributed in part to weight loss, but other mechanisms could be involved such as inhibition of gallbladder contraction and biliary motility [22]. Frequency of acute pancreatitis is marginally elevated with OD liraglutide 3.0 mg (1.3% vs 1.0 in placebo) [21], and similar to placebo in trials of OW semaglutide 2,4 mg in STEP 1 to 4 trials [11-14].
Hypoglycemia
Consistent with the glucose-dependent action of GLP-1 RAs, frequency of hypoglycemia was similar to placebo in patients without diabetes. However, in obesity trials including patients with type 2 diabetes, frequency and severity of hypoglycemia were increased with use of OD liraglutide 3.0 mg (87 versus 31 events per patients-year with placebo) [16]. These hypoglycemia events occurred mainly in patients using sulfonylureas [16]. In STEP 2 trial, severe or blood-glucose confirmed symptomatic hypoglycemia occurred in 5.7% and 3.0% of patients receiving OW semaglutide 2.4 mg and placebo, respectively [12].
Safety Concerns about Liraglutide and Semaglutide
There was numerical increase in breast neoplasms in association with OD liraglutide 3.0 mg. Thus, 10 premalignant and malignant neoplasms were reported in 9 women in the liraglutide arm versus none in the placebo arm [21]. In STEP 4 trial of OW semaglutide 2.4 mg, 3 breast cancers were diagnosed in women randomized to semaglutide versus none in the placebo group [13]. Worsening diabetic retinopathy seems to be an adverse effect specific to semaglutide which was initially observed in association with use of OW semaglutide 0.5-1.0 mg [23]. In STEP 2, there was a trend towards increase in incidence of retinal disorder events in the 2 semaglutide arms compared with the placebo arm [12]. Thus, these events occurred in 6.9%, 6.2%, and 4.2% in patients randomized to OW semaglutide 2.4 mg, OW semaglutiude 1.0 mg, and placebo, respectively [12].
Appraisal of Liraglutide and Semaglutide
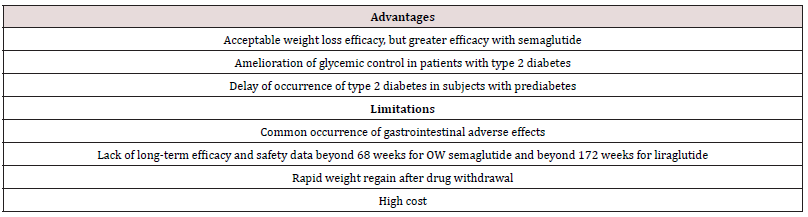
Although available data suggest that OW semaglutide 2.4 mg may be more effective than daily liraglutide 1.8 mg in weight reduction, both drugs offer several advantages for management of obesity. First, their short-term efficacy and safety are supported by well-designed randomized trials [11-15]. Second, being also wellstudied as anti-diabetic drugs, they may be particularly useful in obesity-related type 2 diabetes by causing reduction of both body weight and hyperglycemia [12]. Furthermore, in individuals with pre-diabetes, they delay the onset of type 2 diabetes and increase reversion to normoglycemia [15,21]. The OW administration of semaglutide might virtually enhance compliance with prolonged use. However, both agents have several limitations. First, the common occurrence of GI adverse effects which not uncommonly lead to drug discontinuation. Second, safety beyond 58 weeks is not available for OW semaglutide 2.4 mg [11-14]. The ongoing STEP 5 may in part clarify this problem as it extends over a 2-year period [5]. In case of OD liraglutide 3.0 mg, safety data from placebocontrolled trials are overall reassuring and extend up to 172 weeks [21]. Third, the durability of the weight loss effect is still unclear. In fact, maximum weight loss with use of either drug was achieved after approximately 52 weeks followed by a gradual rebound [11- 15, 21]. Moreover, after drug cessation, weight regain takes place at a more rapid pace along with rise of systolic blood pressure and glycemic parameters to their baselines [14,16]. Hence, these drugs will be taken for years, or even decades as long as weight loss is desired. It is crucial therefore to establish their long-term safety. Fourth, drug cost is another limitation. Advantages and limitations of both agents are summarized in Table 3.
Conclusions and Current Directions
Available clinical trials suggest that OW semaglutide 2.4 mg as an adjunct to healthy life-style changes may be more effective than OD liraglutide 3.0 mg in terms of weight reduction, but not glycemic control. While no head to head comparison is available yet, data derived from respective trials of liraglutide and semaglutide showed superior weight loss with use of OW semaglutide 2.4 mg. Furthermore, head to head comparison of the 2 drugs used in different doses or formulations, consistently showed greater weight loss associated with the use of semaglutide than with liraglutide. However, the superiority of OW semaglutide 2.4 mg will only be confirmed by direct head to head comparison with OD liraglutide 3.0 mg in the setting of randomized, double-blind and double-dummy trials. The possible increase in incidence of breast cancer in association with these 2 agents must be clarified in long-term studies and post-marketing investigations. Similarly, risk of worsening of diabetic retinopathy in relation to the use of semaglutide should be carefully examined. Whereas both drugs in their anti-diabetic doses may reduce CV events in patients with type 2 diabetes, it is equally important to assess their impact on CV outcomes when used in their higher doses for treatment of obesity. In this regard, the SELECT study is an ongoing, double-blind placebo-controlled trial specifically designed to examine the effect of OW semaglutide 2.4 mg on CV outcomes in overweight and obese persons with established CV disease who do not have diabetes [17]. SELECT study started in November 2018 and is expected to recruit 17,500 participants, and last for approximately a total of 59 months.
Read more About This article: https://lupinepublishers.com/diabetes-obesity-journal/fulltext/semaglutide-versus-liraglutide-for-treatment-of-obesity.ID.000162.php
Read more Lupine Publishers Google Scholar Articles : https://scholar.google.com/citations?view_op=view_citation&hl=en&user=nqY8h-kAAAAJ&citation_for_view=nqY8h-kAAAAJ:-f6ydRqryjwC